自己紹介・研究目的
令和6年10月入学/ ■SPRING事業 採択学生紹介

先端薬科学プログラム
令和6年10月 大学院入学
羅 松娟
ラ マツエン
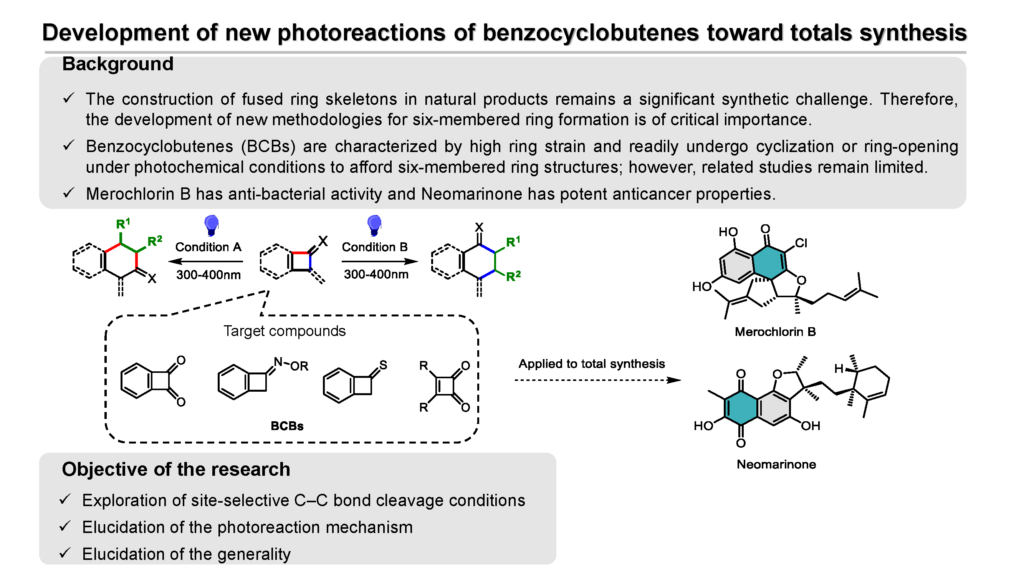
Development of new photoreactions of benzocyclobutenes toward totals synthesis
I am Songjuan Luo, a first-year PhD student at the Graduate School of Medicine and Pharmaceutical Sciences. The title of my research is the development of new photoreactions of benzocyclobutenes toward totals synthesis.
Many natural products are known for their unique structures and specific biological activities. They are important sources of drugs. However, natural extracts suffer from low yields and resource constraints, and chemical synthesis can overcome these constraints and provide stable access to large quantities of products for drug development and production. Merochlorin B has anti-bacterial activity and Neomarinone has potent anticancer properties. These natural products have a common six-ring structure. However, the synthesis of natural products with fused ring skeletons is a challenging task; therefore, the development of new synthetic methodologies remains important. Benzocyclobutenes (BCBs) are characterized by high ring strain and readily undergo cyclization or ring-opening under photochemical conditions to afford six-membered ring structures; however, related studies remain limited.
Building on this, my goal is to investigate the position-selectivity of C-C bond breaking in BCB under different light (300-400 nm) conditions and the influence of various BCB derivatives. This new reaction can promote cycloaddition or ring-opening reactions of high-tensile BCBs, improve regioselectivity and stereoselectivity, achieve complex molecular constructs under mild conditions, and extend the diversity of derivatives for applications in drug synthesis and advanced materials. This research aims to apply the developed photoreactions to the total synthesis of natural products and accelerate natural product-based drug development.
Many natural products are known for their unique structures and specific biological activities. They are important sources of drugs. However, natural extracts suffer from low yields and resource constraints, and chemical synthesis can overcome these constraints and provide stable access to large quantities of products for drug development and production. Merochlorin B has anti-bacterial activity and Neomarinone has potent anticancer properties. These natural products have a common six-ring structure. However, the synthesis of natural products with fused ring skeletons is a challenging task; therefore, the development of new synthetic methodologies remains important. Benzocyclobutenes (BCBs) are characterized by high ring strain and readily undergo cyclization or ring-opening under photochemical conditions to afford six-membered ring structures; however, related studies remain limited.
Building on this, my goal is to investigate the position-selectivity of C-C bond breaking in BCB under different light (300-400 nm) conditions and the influence of various BCB derivatives. This new reaction can promote cycloaddition or ring-opening reactions of high-tensile BCBs, improve regioselectivity and stereoselectivity, achieve complex molecular constructs under mild conditions, and extend the diversity of derivatives for applications in drug synthesis and advanced materials. This research aims to apply the developed photoreactions to the total synthesis of natural products and accelerate natural product-based drug development.