自己紹介・研究目的
令和4年度修了/ ■SPRING事業 採択学生紹介

ナノ新機能物質科学専攻
令和4年度 大学院入学
趙 恒
チョウ コウ
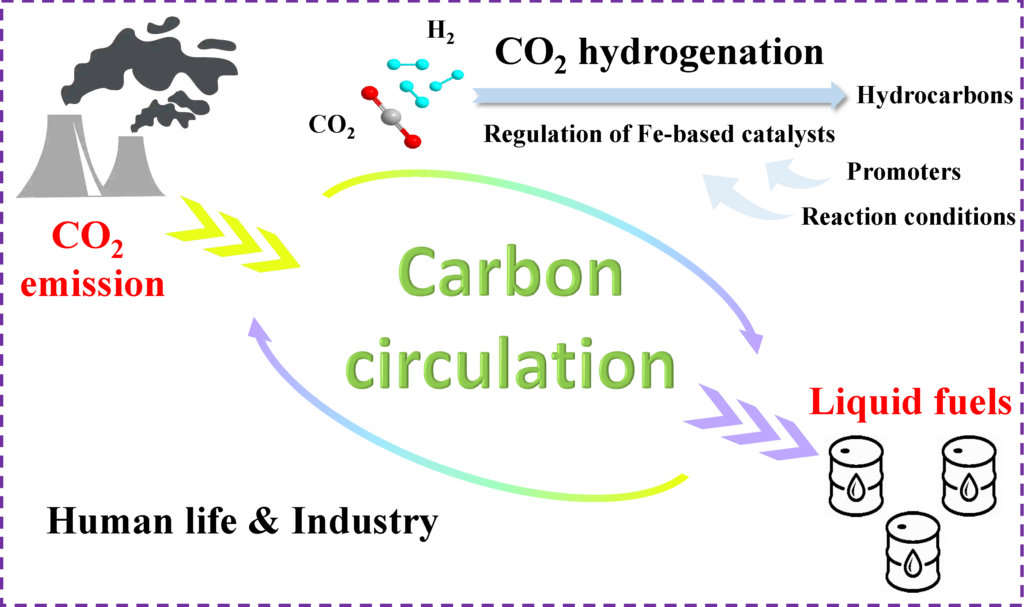
二酸化炭素からの液体燃料合成
Hello everyone, I am Heng Zhao and my major is Nano and Functional Material Science, Graduate School of Science and Engineering. At present, I am studying for my PhD in Tsubaki lab. My research topic is conversion of carbon dioxide (CO2) into liquid fuels.
Owing to the development of industrialization, the demands for carbon-based energy resources have increased rapidly and the corresponding massive emissions of carbon dioxide bring a lot of problems associating with greenhouse effect and ocean acidification. Therefore, trapping, storing and reutilizing CO2 emissions from industry is needed urgently to achieve global carbon neutrality. However, it is a challenge to break the energy barriers caused by the high thermodynamic stability of CO2. Fortunately, thermocatalytic hydrogenation, which is widely researched on converting CO2 into liquid fuels, can conquer the above problem and alleviate energy shortage effectively. Generally, catalytic hydrogenation of CO2 can be realized via the following steps: CO2 is first reduced to CO by reverse water gas shift (RWGS) reaction and subsequently CO is hydrogenated to hydrocarbons via Fischer-Tropsch synthesis (FTS). Obviously, the well-matching catalysis between RWGS and FTS can lead to an improved catalytic result. As an active metal in traditional FTS, iron (Fe) has been widely studied for CO2 hydrogenation. However, pure Fe catalyst generally exhibits low CO2 conversion and high selectivity of undesired methane (CH4). In order to achieve global carbon neutrality and clarify the relationship between physicochemical properties (such as textural properties, surface composition properties, reducibility and surface chemical adsorption capacity) and activity (such as CO2 conversion and products distribution) of the catalyst, my research focuses on the regulation of Fe-based catalysts by introducing various promoters and optimizing reaction conditions for outstanding catalytic performance in CO2 hydrogenation.
Owing to the development of industrialization, the demands for carbon-based energy resources have increased rapidly and the corresponding massive emissions of carbon dioxide bring a lot of problems associating with greenhouse effect and ocean acidification. Therefore, trapping, storing and reutilizing CO2 emissions from industry is needed urgently to achieve global carbon neutrality. However, it is a challenge to break the energy barriers caused by the high thermodynamic stability of CO2. Fortunately, thermocatalytic hydrogenation, which is widely researched on converting CO2 into liquid fuels, can conquer the above problem and alleviate energy shortage effectively. Generally, catalytic hydrogenation of CO2 can be realized via the following steps: CO2 is first reduced to CO by reverse water gas shift (RWGS) reaction and subsequently CO is hydrogenated to hydrocarbons via Fischer-Tropsch synthesis (FTS). Obviously, the well-matching catalysis between RWGS and FTS can lead to an improved catalytic result. As an active metal in traditional FTS, iron (Fe) has been widely studied for CO2 hydrogenation. However, pure Fe catalyst generally exhibits low CO2 conversion and high selectivity of undesired methane (CH4). In order to achieve global carbon neutrality and clarify the relationship between physicochemical properties (such as textural properties, surface composition properties, reducibility and surface chemical adsorption capacity) and activity (such as CO2 conversion and products distribution) of the catalyst, my research focuses on the regulation of Fe-based catalysts by introducing various promoters and optimizing reaction conditions for outstanding catalytic performance in CO2 hydrogenation.