自己紹介・研究目的
令和5年度修了/ ■SPRING事業 採択学生紹介

ナノ新機能物質科学専攻
令和5年度 大学院入学
梁 嘉鳴
リヨウ カ メイ
Direct conversion of CO2 to aromatics over K-Zn-Fe/ZSM-5 catalysts via a Fischer-Tropsch synthesis pathway.
Hello, everyone. Please let me introduce myself. My name is Jiaming Liang. I come from China and i am a second-year doctor student in Tsubaki Lab. My major is Nano and Functional Material Science. In recent years, CO2 has attracted more and more attention as a greenhouse gas. How to convert the environmentally harmful CO2 gas into high value-added chemical products has become a very urgent question. As a result my research topic is converting CO2 into aromatics which are very important chemical products. I think it is a very significant project for the environment and human. Thank you very much.
CO2 is an appropriate raw material for organic synthesis from the standpoint of environmental conservation because it is an inexpensive and abundant carbon source. CO2 hydrogenation to form basic chemicals such as methanol and hydrocarbons has attracted considerable attention in the past decades.
Benzene is a chemical prepared mostly from petroleum and coal tar, and can be used to produce industrial chemicals. As crude oil becomes scarcer, there is an urgent need for a new method to produce benzene. More recently, bifunctional catalysts by coupling Fischer-Tropsch synthesis (FTS) catalysts and zeolite catalysts achieved a promising results.
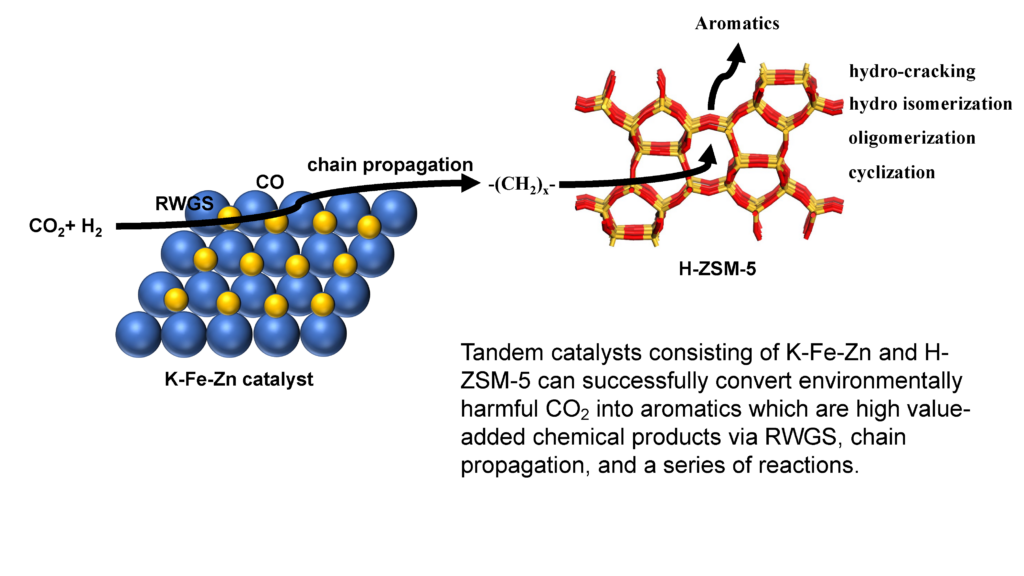
Iron-based catalysts are ideal options for CO2 conversion via a modified FTS due to its catalytic capacity in both the RWGS and FTS reactions. Under working reaction conditions, Fe-based catalysts contain two parts of active sites: Fe3O4 for CO intermediate production and Fe5C2 for subsequent chain propagation. On the other hand, H-ZSM-5 zeolites with suitable pore shapes have been employed as ideal catalysts for aromatics formation from methanol or alkenes. As a result, tandem catalysts consisting of K-Fe-Zn and H-ZSM-5 can successfully convert environmentally harmful CO2 into aromatics which are high value-added chemical products via RWGS, chain propagation, and a series of reactions.
CO2 is an appropriate raw material for organic synthesis from the standpoint of environmental conservation because it is an inexpensive and abundant carbon source. CO2 hydrogenation to form basic chemicals such as methanol and hydrocarbons has attracted considerable attention in the past decades.
Benzene is a chemical prepared mostly from petroleum and coal tar, and can be used to produce industrial chemicals. As crude oil becomes scarcer, there is an urgent need for a new method to produce benzene. More recently, bifunctional catalysts by coupling Fischer-Tropsch synthesis (FTS) catalysts and zeolite catalysts achieved a promising results.
Iron-based catalysts are ideal options for CO2 conversion via a modified FTS due to its catalytic capacity in both the RWGS and FTS reactions. Under working reaction conditions, Fe-based catalysts contain two parts of active sites: Fe3O4 for CO intermediate production and Fe5C2 for subsequent chain propagation. On the other hand, H-ZSM-5 zeolites with suitable pore shapes have been employed as ideal catalysts for aromatics formation from methanol or alkenes. As a result, tandem catalysts consisting of K-Fe-Zn and H-ZSM-5 can successfully convert environmentally harmful CO2 into aromatics which are high value-added chemical products via RWGS, chain propagation, and a series of reactions.